Which Best Describes Elevctrolytes and Nonelectrolytes in Solutions
A solution may be a gas solid or liquid. As a result electrolyte solutions readily conduct electricity.

Electrolytes And Nonelectrolytes Chemistry For Non Majors
An aqueous solution is made from a molecular compound rather than from an ionic compound.

. You know that one of them is a suspension one is a solution with nonelectrolytes and the other is a solution with electrolytes. Ionic compounds are generally electrolytes. 3 It has a mass of 0 and a charge of 0.
We review their content and use your feedback to. Electrolyte and Nonelectrolyte Solutions. 2 It has a mass of 0 and a charge of 1.
Experts are tested by Chegg as specialists in their subject area. 1 Matter is converted to energy. Solutions of Electrolytes Nonelectrolytes.
29 Which statement best describes gamma radia-tion. As a result electrolyte solutions readily conduct electricity. From this observation you can conclude the solution is probably.
Solutions Electrolytes And Nonelectrolytes Lab ReportElectrolytes are salts or molecules that ionize completely in solution. 4 It has a mass of 4 and a charge of 2. Which of the following concerning electrolytes and nonelectrolytes isare true.
As a result electrolyte solutions readily conduct electricity. This statement is not true because They disperse as ions not as molecules. 1 It has a mass of 1 and a charge of 1.
Electrolytes yield ions in solution non-electrolytes do not. A weak electrolyte a nonelectrolyte a strong electrolyte a covalent electrolyte. Nonelectrolyte solutions do not therefore conduct electricity.
NON ELECTROLYTES do not dissociate into ions in solution. A solution is a homogeneous mixture of two or more substances. In water acetic acid is a weak electrolyte because at normal temperature it only dissociate 5.
Hope you get the answer report flag outlined. People also ask what is a Nonelectrolyte. Question from discovery education.
1 and 3 1. The ability to form an electric current. Electrolytes in Body Fluids.
Electrolyte solutions can conduct electricity. The expression like dissolves like describes the polaritiesof the solute and solvent particles needed to form a solution. ONa 2 SO 4 s 2Naaq SO 4.
Solutions that dissolve in water but dont conduct electricity are nonelectrolytes. Nonelectrolyte solutions do not therefore conduct electricity. Some molecular substances are electrolytes.
2 Energy is converted to. Learn more about weak electrolytes with this lesson called Weak Electrolyte. Causes solute to dissolve dissociate if electrolyte and have pH-dependent dissociation for weak acids and bases solute.
A compound that does not conduct an electric current in either aqueous solution or in the molten state. If some ions are present in solution which best describes the solute. A solution is a heterogeneous mixture of two or more substances.
You are given three liquids. Lowers solvent vapor pressure elevates solvent boiling point depresses solvent freezing point induces osmotic pressure from solvent. The University of the State of New York REGENTS HIGH SCHOOL EXAMINATION PHYSICAL SETTING CHEMISTRY Friday June 21 2002 115 to 415 pm only You are to answer all questions in all parts of this examination according to the directions provided in the examination booklet.
Describe the process by which you would differentiate between the three. 30 Which change takes place in a nuclear fusion reaction. Nonelectrolytes do not dissociate into ions in solution.
When the ends of two wires from a circuit containing a battery and a lightbulb are placed into a beaker containing an aqueous solution the light bulb glows brightly. Which of the following best describes a solution Select one. C 12 H 22 O 11 s C 12 H 22 O 11 aq H 2.
Some molecular substances are electrolytes. All electrolytes are ionic substances. ELECTROLYTES are salts or molecules that ionize completely in solution.
A nonelectrolyte is a substance that does not exist in an ionic. Strong electrolytes partially ionize in solution. A concentrated but weak base.
Who are the experts. A compound that conducts an electric current when it is in an aqueous solution or melted. Strong electrolytes partially ionize in solution.
Electrolytes disperse as molecules in solution. All electrolytes are ionic substances. If the solution contains a weak electrolyte like 5 acetic acid the bulb will be dim.
A solution is any mixture of two or more substances. Advantages of solutions as oral dosage forms. This lesson will adhere to the following.
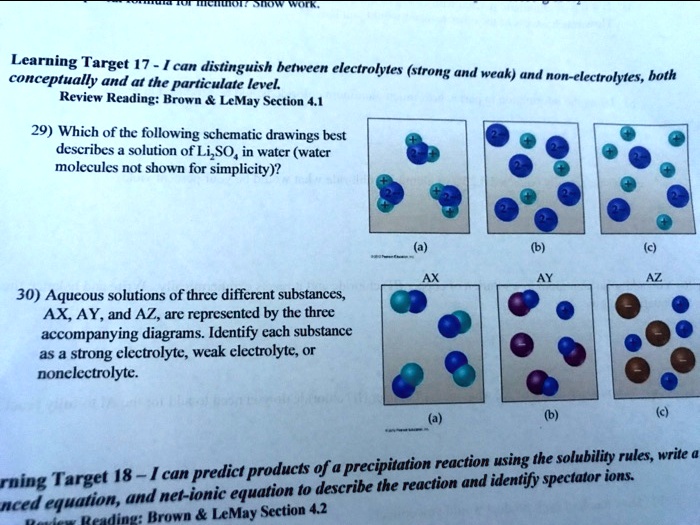
Solved Learning Target 17 Can Distinguish Between Electrolytes Strong And Weak And Conceptually And At The Particulate Level Non Electrolytes Hoth Review Reading Brown Lemay Section 4 1 29 Which Of The

No comments for "Which Best Describes Elevctrolytes and Nonelectrolytes in Solutions"
Post a Comment